this paper is from a laboratory experiment in which I utilize various laboratory procedures that every organic chemistry student should be familiar with he really liked this one alot
17. October 1996
Experiment #7
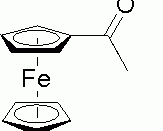
Acetylation of Ferrocene
Introduction
In this lab we will be utilizing the Friedel Crafts process of acetylation of ferrocene. Ferrocene is an atom of iron bounded by two aromatic rings. We will use some reagents that will cause the ferrocene to add either one acetyl group to an aromatic ring or add two acetyl groups to each of the aromatic rings. In order to determine how well this process had worked we employed: IR spectra analysis, column chromatography, and a little TLC. This experiment is relevant in today's highly industrialized world. By utilizing many of the techniques we employ in this lab, a company can synthesize new types of materials or composites that could revolutionize an industry.
Background
When we react the ferrocene with phosphoric acid and acetic anhydride, we obtain many disparate products. Not only do we get acetylferrocene, but we also get diacetylferrocene, some unreacted ferrocene reactant, and acetic acid as well. We will use thin layer chromatography (TLC), column chromatography (CG), and IR spectra analysis in order to determine the what proportions of each of these compounds will be present in the final product.
Both TLC and CG are excellent methods of measuring the presence of a given substance. Both methods turn around a compounds polarity. As one recalls, polarity is a measure of the electronegativity of a compound determined by their placement in the periodic chart. Specifically, in this lab we are talking about the difference in polarity between the atoms of oxygen and carbon. Ferrocene is relatively low to none in polarity. Acetylferrocene, because of the carbonyl functional group, is more...