Acid comes from the Latin word acere, which means sour. In dilute concentrations acids are responsible for the sour taste of lemons, limes, vinegar and other substances. Hydrochloric acid is produced in your stomach to help digest food. Sulfuric acid is found in car batteries. Acids can conduct an electric current. Acids are also corrosive. When acids react with metals they form hydrogen gas. When an acid reacts with a carbonate it forms carbon dioxide, water, and a salt. Acids can cause skin to burn so you should be careful when handling acids.
Our understanding of acids began with Michael Faraday's discovery in 1834 that acids are electrolytes. When they are dissolved in water they produce a solution that contains ions and can conduct electricity. In 1884 Svante Arrhenius proposed that an acid be defined as a hydrogen-containing compound that when dissolved in water produces a concentration of hydrogen ions greater than that of pure water.
There has been criticism of his theory because it applies only to water solutions while many acid-base reactions are known to take place in the absence of water.
Johannes Bronsted and Thomas Lowry proposed a better theory in 1923. Their theory states that an acid is a proton donor. Although the acid must still contain hydrogen, the Bronsted-Lowry theory does not require a water medium. The Bronsted-Lowry theory also explained why a strong acid displaces a weak acid from its compounds.
Gilbert Lewis has offered another theory that has the advantage of not requiring the acid to contain hydrogen. This theory states that acids are electron-pair acceptors. This theory also has the advantages that it works when solvents other than water are involved and it does not require the formation of a salt.
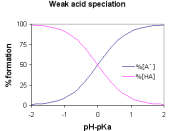
One way to classify acids is as strong and weak. Their...
![Copper mining and sulfuric acid plant, Copperhill], Tenn. (LOC)](https://s.writework.com/uploads/3/32663/copper-mining-and-sulfuric-acid-plant-copperhill-tenn-loc-thumb.jpg)