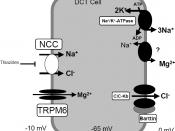
Due to the nature of amphotericity and the conformation of plasma membrane, large or ionic molecules can not permeate through plasma membrane freely. To help with those transportations, protein involved transports are adopted. One of which is active transport, that uses energy to move substrates against electrochemical gradient. Among many active transport cases, sodium potassium transportation has been most widely studied for it is expressed in virtually all cells of higher organisms (Austgen 2003).
The Na,K-ATPase, a P-type ATPase family member (Kaplan 2002), also known as sodium pumper (Kaplan 2002), is the transporter protein that exchanges sodium and potassium. Na, K-ATPase is composed of two subunits: ñ-subunit and ò-subunit (Kaplan 2002). Different ATPases vary with their ñ-subunit. Depending on cellular environment and cell function, there are four ñ-subunit isoforms: ñ1, ñ2, ñ3, and ñ4 (Takahashi et al. 2004), and different forms of ñ-subunit are encoded from different genes and with minor difference on amino acid sequences (Kaplan 2002).
The mechanism of Na-K active transport has been widely studied, and the over all reaction can be described as by consuming energy retrieved from hydrolyzing one ATP, three intracellular sodium ions are exchanged with two extracellular potassium ions.
Study has shown that the ATP hydrolysis caused by sodium potassium active transport varies a lot. In resting human cells, only 25% of all cytoplasmic ATP is hydrolyzed by sodium potassium transport, whereas in nerve cells such as lens cells, the number is as high as 70% (Austgen 2003). The reason for this discrepancy is suspect to be the difference of Na-K ATPase activity. Even in cells from a same organ, the activities vary a lot: epithelium and fiber, two cell types in the lens, have very different Na-K ATPase activities, and the activity is much higher in the epithelium (Delamere 2003). However, experimental...