1. Abstract
This experiment implemented a Friedel-Crafts acylation in order to acylate anisole with acetic anhydride in the presence of anhydrous aluminum chloride and dichloromethane solvent. The acylation produced para- Methoxyacetophenone. The experiment implemented gentle reflux, extraction, rotovap, and H1 NMR/ IR spectrum analysis. The experiment yielded an oily, clear substance weighing 0.221 grams (14.7% recovery). The H1 NMR spectrum showed a 1:1 ratio between Ha and Hd, verifying the reaction took place; there were hydrogens present from both anisole and acetic anhydride in para- Methoxyacetophenone (methoxy and acyl group, respectively). The H1 NMR also showed only two types of aromatic hydrogens, verifying the para orientation. The IR spectrum displayed a conjugated ketone, verifying the desired product.
2. Introduction
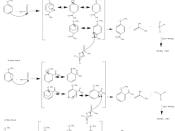
The Friedel- Crafts acylation is an important reaction for "synthesis of aromatic ketones intermediates in the fine chemical and pharmaceutical industry" 1. The reaction installs an acyl group with the help of a Lewis acid 2.
Lewis acids, such as AlCl3, work as catalysts to make better electrophiles by pulling electrons out of the molecule. In the case of acylation, the intermediate is deemed an "acylium ion" 2. After formation of the acylium ion, the aromatic benzene ring acts as a nucleophile and attacks the positive charge. The ring is then deprotonated to restore aromaticity, in typical 2-step electrophilic aromatic substitution fashion 2. The âÂÂâÂÂâÂÂâÂÂrate and regiochemistry of these electrophilic aromatic substitutions depend on substituents present on the benzene. Substituents on a ring can either be activating or deactivating. Activating groups are electron donating; these groups can donate electron density to a ring and stabilize carbocations. By stabilizing carbocations, the entire energy of activation for formation is lower, which is extremely favorable and faster than benzene 2. These activating groups direct ortho and para. Deactivating groups, on the...