Alkanes
Physical properties:
The chemistry of carbon compounds is called organic chemistry. There are millions of organic chemicals, but they can be divided into groups called homologous series. All members of a particular series will have similar chemical properties and can be represented by a general formula.
The alkane series is the simplest homologous series. The main source of alkanes is from crude oil.
Alkanes are covalent compounds. They are hydrocarbons, which means they contain hydrogen and carbon. The general formula for an alkane is .
Properties and uses of alkanes:
Name of alkane: Melting point oC: Boiling point oC: Density g/cm3: State at room temperature:
Methane CH4 -182 -162 0.42 Gas
Ethane C2H6 -183 -88 0.55 Gas
Propane C3H8 -188 -42 0.58 Gas
Octane C8H18 -57 126 0.72 Liquid
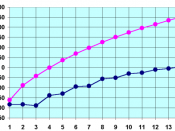
The first four alkanes are gases at room temperature.
Alkanes with 5-17 carbon atoms are liquids.
Alkanes with 18 or more carbon atoms are solids.
As the number of carbon atoms increases, the melting points, boiling points and densities increases.
They are insoluble in water but dissolve in organic solvents such as benzene.
Their chemical reactivity is poor. The C-C bond and C-H bond are very strong so alkanes are not very reactive.
They will carry out combustion. Burning alkanes in air (oxygen) produces water and carbon dioxide. The reactions are very exothermic (give out heat energy), so alkanes in crude oil and natural gas are widely used as heating fuels.
For example:
If alkanes combust in too little air, carbon monoxide may form. This is dangerous and can cause death.
Cracking alkanes
The lighter fractions (for example, petrol) are in large demand. The heavier fractions are not so useful but unfortunately chemists have to be able to convert these heavier fractions into petrol and other useful products, due...