Amedeo Avogadro was born on 9th August 1776 in Turin, Italy. He graduated to become a lawyer but later he became interested in science. In 1800 he began his private studies in science and mathematics. In 1809 he started teaching science in high school in Vericelli. It was in Vericelli that he published all his papers regarding masses and densities of gases and formulated the hypothesis which is now knows as the Avogadro Law.
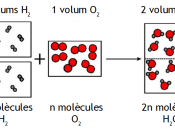
In 1811 Avogadro published an article showing the difference between a molecule and an atom. Other scientist like Dalton had got confused and believed that a molecule and atom are the same. Avogadro clarified that two atoms of gases like hydrogen and oxygen formed one molecule of each gas. Avogadro further formulated Avogardo's Principle which stated that equal volumes of all gases at the same temperature and pressure contain the same number of molecules. This statement was of great importance.
other scientists like Cannizarro used this statement to determine atomic weights, molecular weights of various gases and the number of molecules in molar weights of the gases. Cannizarro also determined the constant number of molecules in molar weights of the gases to be exactly 6.022 x 1023. This number was called Avogadro Number in the honour of the scientist.
Before Avogadro's time, the qualitative basis of chemistry was well known but quantitive basis was not clear. For example, it was known that chlorine and hydrogen will react to form hydrochloric acid but it was not clearly known how much hydrogen was needed to react fully with certain amount of chlorine and how much hydrochloric acid will be produced. Avogadro's researched helped exact determination of quantities of different gases which will be required to fully react with each other and also the exact quantity of the new...