Experiment V03 Analysis of commercial vitamin C tablets
Chemicals: Vitamin C tablet, (1)
standard 0.0110 M potassium iodate (KIO3) solution, (100 cm3)
standard 0.060 M sodium thiosulphate solution, (Na2S2O3, 160 cm3)
1 M potassium iodide solution, (KI, 20 cm3)
0.5 M H2SO4, (150 cm3)
freshly prepared starch solution.
Apparatus: Titration apparatus
Principle: In this experiment you are required to determine the vitamin C content of a commercial tablet and compare this with the manufacturer's specification.
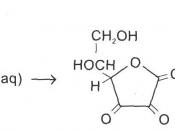
Ascorbic acid
The standard method for determination of ascorbic acid involves the direct titration of acidified sample with a standard iodine solution. But the low solubility of iodine makes this procedure less than ideal.
The proposed experiment avoids these difficulties is by using the reaction between iodide (in excess) and iodate which generate a known excess quantity of iodine, and this excess iodine is back titrated with standard sodium thiosulphate solution.
The reactions are as follows:
IO3-(aq) + 5I-(aq) + 6H+(aq) (((( 3I2(aq) + 3H2O(l)
I2(aq) + 2S2O32-(aq) (((( 2I-(aq) + S4O62-(aq)
P.1
Experiment V03 Analysis of commercial vitamin C tablets
Procedure:
Dissolve the vitamin C tablet provided (Roche Vitamin C effervescent tablet, claimed to contain 1 g of ascorbic acid) in about 150 cm3 of 0.5 M sulphuric acid.
Transfer the resulting solution to a clean volumetric flask and make up to 250 cm3 using distilled water.
Pipette 25.0 cm3 of the vitamin C solution into a conical flask and add to it 5 cm3 of 1 M potassium iodide solution. Then pipette 25.0 cm3 of the standard KIO3 solution into the flask containing vitamin C and potassium iodide. The excess iodine is immediately back titrated with the...