The Chemical Context of Life
I. Chemical Elements and Compounds
A. Matter consists of chemical elements in pure form and combinations called compounds.
1. Matter is anything that takes up space and has mass.
2. An element is a substance that cannot be broken down into other substances by chemical reactions.
a. Every element has its own symbol.
b. There are 92 naturally occurring elements.
3. A compound is a substance consisting of two or more elements combined in a fixed ratio.
a. A compound has characteristics beyond those of its combined elements.
B. Life requires about 25 chemical elements.
1. The four main elements known to be essential to life are carbon, oxygen, hydrogen, and nitrogen.
a. These four make up 96% of living matter.
2. Trace elements are those required by an organism in only minute quantities.
a. Iron is an example of a trace element that is required by all forms of life.
II. Atoms and Molecules
A. Atomic structure determines the behavior of an element.
1. An atom is the smallest unit of matter that still retains the properties of an element.
a. Each element consists of a certain kind of atom that is different from the atoms of any other element.
b. Atoms are symbolized with the same abbreviation used for the element made up of those atoms.
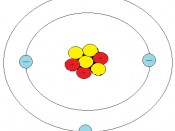
B. Subatomic Particles
1. There are three kinds of particles: neutrons, protons, and electrons.
a. Neutrons and protons are packed together tightly to form an atomic nucleus at the center of the atom.
b. The electrons form a cloud around the nucleus, moving at nearly the speed of light.
c. Each electron has one unit of negative charge.
d. Each proton has one unit of positive charge.
e. A neutron is electrically neutral.
f. Neutrons and protons...


Apology
Sorry about the "t;Tab/>" throughout the outline. Does anyone know how to keep this from happening? If you need to use this outline, a useful way of eliminating these would be doing Replace(Ctrl + H in Word) and replacing t;Tab/> with .
0 out of 0 people found this comment useful.