Aspirin
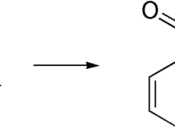
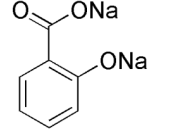
Aspirin is a white crystalline substance made of carbon, hydrogen, and oxygen. It is used in the treatment of rheumatic fever, headaches, neuralgia, colds, and arthritis; reduce temperature and pain. The formula for aspirin is CH3CO2C6H4CO2H. Aspirin's scientific name is actylsalicylic acid (ASA). The main ingredient in ASA is salicylic acid. This ingredient grows in small roots, leaves, flowers and fruits on plants.
About 100 years ago, a German chemist, Felix Hoffmann, set out to find a drug that would ease his father's arthritis without causing severe stomach irritation that came from sodium salicylate, the standard anti-arthritis treatment of the time. Hoffmann figured that the acidity of the salicylate made it hard on the stomach's lining. He began looking for a less acidic formulation. His search led him to the synthesization of acetylsalicylic acid. The compound shared the therapeutic properties of other salicylates, but caused less stomach irritation.
ASA reduced fever, relieved moderate pain, and, at higher doses, alleviated rheumatic fever and arthritic conditions.
Though Hoffmann was confident that ASA would prove more affective than other salicylates, but his superiors incorrectly stated that ASA weakens the heart and that physicians would not subscribe it. Hoffmann's employer, Friedrich Bayer and Company, gave ASA its now famous name, aspirin.
It is not yet fully known how aspirin works, but most authorities agree that it achieves some of its effects by hindering the flow of prostaglandins. Prostaglandins are hormone-like substances that influence the elasticity of blood vessels. John Vane, Ph. D., noted that many forms of tissue injury were followed by the release of prostaglandins. It was proved that prostaglndins caused redness and fever, common signs of inflammation. Vane's research showed that by blocking the flow of prostaglandins, aspirin prevented blood from aggregating and forming blood clots.
Aspirin can be used...
Aspirin
Very informative essay
1 out of 1 people found this comment useful.