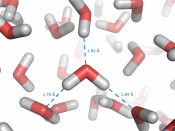
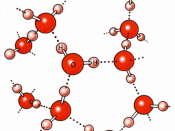
Basic concept and example in entropy.
An endothermic process with a loss of entropy: (An Reaction which absorbed energy and result formation of more order substances/compounds)
Recrystalistaion:
In order to dissolved the crystals in the solvent, energy is required to be put into system in the form of heat, (increasing the kinetic energy of the system to dissolved the impure crystals .As the kinetic energy of the system increased,( the molecules within the solvent absorbed the energy and moved with increased energy and collision between the solvent molecules and the crystal increases,(since the solvent molecules is trying to dissipated the kinetic energy if has absorbed) eventually the energy supplied will be greater than that of bonds energy (the lattice energy which hold the crystal together) within the crystal. The lattice will eventually be broken resulting in the crystal existing as ions within the solution. The impurity originally trapped within the crystal is separated from the crystal, (the crystal structure is now broken and exists as free ions within the solution)
The impurity is then filtered off, then the crysta ions are now free to recrystallise out of the solution.
As the crystal is reformed, bonds are also reformed between crystals giving out energy and in the process becoming more stable (as a result the system has a net loss in entropy)
The net energy change in terms of energy supplied and given out, as the bonds between the crystals are broken and reformed. The amount of energy (heat) supplied to the system is greater than that of the energy (heat) given out as the crystal lattice is reformed. (Therefore the reaction is endothermic)
An endothermic process with a gain in entropy (A reaction which absorbed heat and result in a less ordered/more disorder substance):
Phase change of water from liquid...