Irons periodic identification is "FE". Iron has an atomic mass of 55.845 AMU and its atomic number is 26. The melting point for iron is 1535.0 ðC (1808.15 ðK, 2795.0 ðF) and its boiling point is 2750.0 ðC (3023.15 ðK, 4982.0 ðF). There are 26 protons and electrons and 30 neutrons. Iron is a transition metal and its crystal structure is cubic. Iron has a density of 7.86 g/cm3 when measured at a level of 293 K. The isotopes of iron are FE-52, FE-54, FE-55, FE-56, FE-57, FE-58, FE-59, and FE-60.
The date of discovery isn't very specific. It has a Latin origin and its term is ferrum. Iron is a basis for many uses. It is often used in hemoglobin, multi-vitamins and steel. Iron is also a supplementary cure to a common case of Anemia. Steel originated in Pittsburgh, Pennsylvania in 1875. This newfound idea, started out to be a cheap investment by Andrew Carnegie supported by The Edgar Thomson Works.
Carnegie saw a potential in steel for railways, the iron in rail tracks had been wearing away too quickly and causing terrible episodes. Carnegie soon rid of his job to manufacture this idea to make safer rails from the United States to England. As profits increased Carnegie hired railroad engineers for mass production. A steel plant soon came about with hot furnaces, called blast furnaces and rolling mills in machine shops. Steel was in such a high growth point that more plants developed and communities were urbanized around steel and all of its properties and aspects.
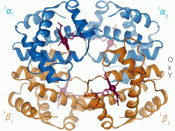
Hemoglobin is a macromolecule that has four heme complexes and peptide chains. The peptide chains control the proteins such as the portion tetramer. Amino acids can be recognized as a hetero-tetramer. The iron in hemoglobin has a heme complex atom built...


Iron Overload
One common disease caused by iron you didn't mention in your article is iron overload. TOO MUCH IRON WILL CRIPPLE YOU.
5 out of 5 people found this comment useful.