Outline the role of condensation and hydrolysis in the relationship between amino acids and dipeptides. Explain the use of two named enzymes in biotechnology.Water is the molecule made up of hydrogen and oxygen and it plays a key role in an existence of living organisms. It is due to its properties like: transparency, cohesion, solvent properties and thermal properties: heat capacity, boiling and freezing points and the cooling effect of evaporation.
Transparency means that light can pass through the water and reach chloroplasts where it is used in the process of photosynthesis. Cohesion occurs when the water molecules stick to one another because of hydrogen bonding. This water property, called also as a surface tension, acts an important role in as the significance to living organisms as the strong surface film allows small organisms to be attached below or supported above. or example a pond-skater can float or walk on the surface of the water.
Since the water is a great solvent, many substances easily dissolves in it. It happens because water is slightly polar (what means that organic substances such as sodium and organic substances (solutes) such as glucose can dissolve in it. The solvent properties of water allow substances to be carried in blood and cell sap in plants.
Water has its thermal properties as well. Water has a large heat capacity ÃÂ to raise its temperature, we need large amounts of energy because the role of heat is to break the hydrogen bonds. Level of the heat energy is given out as the water is cooled down.
Next of the water thermal properties are the boiling and freezing points. Water boils in natural habits on the Earth in the temperature of 100ðC, what is too high for the living organisms to survive. WaterÃÂs freezing point is relatively high à0ðC. However, because water becomes less dense as it freezes, ice is formed. Ice that forms on the lakeÃÂs surface insolates the water underneath so living things can survive, without the water temperature being too low.
Water is also a good coolant and has evaporation properties. High heat of vaporization gives efficient cooling of the body as sweat evaporates. In plant leaves in happens in terms of transportation. It happens because water can evaporate at temperatures below boiling point.
There are also some physical properties of water that have biological importance for living organisms. For example density - when displaced, water gives good support for aquatic organisms. Capillarity may be involved in transporting water in the steam of a plant. Moreover, the fact that water is incompressible is important in transport systems and as means of support in organisms with a ÃÂhydrostaticÃÂ skeleton. Another water property - called electric conductivity is important for the functioning of many cells such as nerve cells as ions dissolved in water make cytoplasm quite a good conductor.
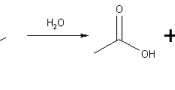
Water has also a significant role in the terms of reactions. It is formed in the condensation reaction, and it is used up to break down some molecules in the hydrolysis reaction. Two amino acids can be joined together and form a dipeptide by a condensation (or dehydration) reaction, where water is also formed. In this reaction the bond between oxygen and hydrogen is broken; the hydrogen joins the hydroxyl group and attach to the oxygen. Then the bond between this oxygen and the carbon is broken and the water molecule thus formed moves away. Then the lone electrons of both oxygen and hydrogen join and form a new covalent bond.
The reverse reaction is also possible and it is called a hydrolysis reaction. Using the water molecules, it breaks large molecules such as polypeptides, polysaccharides and triglycerides, into smaller ones.
Enzymes are widely used in the biotechnology; the technology based on biology that uses organisms or parts of the organisms to produce things to carry out useful processes. For example the use of pectinase in fruit juice industry and the use of protease in biological washing powder.
Pectin is a polysaccharide that is found in the cell walls of plants. Its role is to break down pectin by hydroliysis reactions. The advantage of the process is to gain more juice from fruits and vegetables because pectinase makes juices more fluid and easy to separate from the pulp. It also makes the juice less cloudy by helping the solid suspended in juice to settle down and be separated from the fluid.
Protease is obtained by culturing a bacterium, Bacillus licheniformis. enzymes break down proteins into soluble peptides and amino acids. It is used in laundry washing powders (called the biological powders if they contain this enzyme). Since much of the dirt is made up of protein not lipids, thanks to protease the dirt is removed, because protease digests proteins during the wash. The high pH optimum of the protease helps it to remain active, despite the high pH caused by alkalis in the washing powder. Moreover, protein stains on clothes can only removed by using a very high temperature wash that is not allowed in more delicate cloths. Protease lowers that temperature, lowers the energy needed in washing and eliminates the risk of shrinkage.