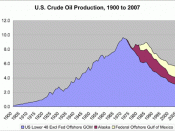
How can we decide how to make the best use of our limited oil supplies?
Crude oil or "unprocessed oil" is a mixture of mostly hydrocarbons and a small percentage of sulphur compounds. It is a fossil fuel as it was initially formed around 300 million years ago, when tiny animals on the sea died and fell to the ocean bed, where sand and mud prevented it from degrading. Over millions of years, the increase of sand and pressure caused the dead animals to become crude oil. Anywhere that crude oil is found was once a sea bed. The oil varies depending from where it's found, having different properties of viscosity and colour.
Crude oil itself isn't very useful; it first must be separated or refined in a process called fractional distillation. In this process, crude oil is heated to temperatures above 500úC along with steam, and all the compounds in the mixture evaporate, except bitumen which remains in the bottom.
As the gas rises, the temperature cools, and as the temperature reaches to about the compound's boiling point, it condenses to form a liquid, and the compounds with different boiling points will remain in separate levels.
Most of the hydrocarbons from crude oil are alkanes, saturated compounds from the same homologous series. Alkanes contain only carbon and hydrogen molecules with single bonds. These are easily separated in fractional distillation, because the longer the molecules of the alkanes, the higher the boiling point will be. Once these fractions are separated, three other process take place in order to obtain different compounds. These are cracking, alteration and unification. The process of cracking is when hydrocarbons with larger molecules are broken down into smaller compounds by applying heat and pressure along with a catalyst. The molecule will often break down to form...