Carbon: Carbon, with symbol C is a non-metallic chemical element. It is a Halogen with the Atomic Number 6 in group IV, period II. Carbon is not very plentiful, it only makes up 0.025% of the Earth's crust, but it still forms more compounds than all of the other elements combined.
Carbon can exist in a variety of different physical forms. These include:
Diamond: Diamond is one of the many allotropes of Carbon. Diamonds are widely known as a mineral with extreme physical qualities - they make excellent abrasives because they are the hardest known mineral. It is a poor conductor of electricity.
Graphite: Graphite is one of the most common allotropes of Carbon. Graphite is a soft, slippery solid that is a good conductor. It is also a very good lubricant (something that reduces friction between two moving surfaces). Graphite is known as being one of the most stable forms of carbon ever discovered.
Graphite is also used for electrodes in electric furnaces and dry cells as well as for making crucibles in which metals are melted.
Lonsdaelite: Lonsdaelite is an allotrope of the Carbon allotrope, diamond. It has a hexagonal shape and is formed when meteoric graphite falls onto the earth's surface. The graphite turns into diamond, but it still withholds the graphite's hexagonal crystal structure.
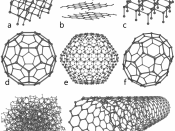
Fullerenes: Fullerenes are molecules which are made entirely out of carbon. Its shape is a hollow sphere, an ellipsoid or a tube. Fullerenes are under the study for medical uses, to target resistant bacteria and even cancer cells.
Amorphous carbon: Amorphous carbon is the name used for carbon that does not have any crystalline structure. Amorphous carbon actually contains crystallites of graphite or diamond with bits of amorphous carbon holding them together.
Carbon nanotube: Carbon nanotubes are cylindrical shaped...


Its Alright...
You could have gotten rid of all the "<Tab/>" for the people wanting to use this, but its great for a year 9 report on "Carbon and its allotropes"...I can imagine how many people would get that specific thing to write on.
0 out of 0 people found this comment useful.