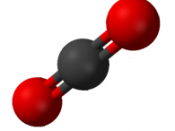
Carbon Dioxide is an unreactive, colourless, odourless gas that occurs in small quantities in the earth's atmosphere naturally. The earth's ocean, soil, plants and animals release CO2. The formula of Carbon Dioxide is CO2. The CO2 molecule contains 2 oxygen atoms that each share 2 electrons with a carbon atom to form 2 carbon - oxygen double bonds. The atoms are arranged as so (OHT). This is called a 'linear molecule'.
Carbon dioxide is commonly found as a gas and is never a liquid. It sublimes to a solid known as 'dry ice' which is used as a substitute for normal ice as it is a lot colder and doesn't melt.
Humans and animals breathe out Carbon Dioxide, often referred to as the greenhouse gas, as a waste product. Plants take in this CO2 and use it to make food. This is called photosynthesis. During this process oxygen is released which is then breathed in by humans and animals.
This procedure is repeated over and over and a natural balance is obtained. However this natural balance is disrupted by human activity. People of the world are putting more than 5.5 billion tons of CO2 into the atmosphere every year. 75% of this is caused from the burning of fossil fuels. These fuels are burnt all the time to run factories, power plants and vehicles. The main sources of CO2 emissions are electric utilities, residential buildings, industry and transportation. The other 25% is induced by the destruction of the world's forests. The reason for this is that there are less trees and plants to take in the CO2 but there is just as many, if not more, humans and animals to breathe it out.
The amount of CO2 in a planet's atmosphere affects the temperature of the planet. As more...