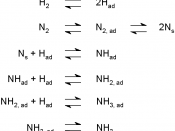A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without itself undergoing any change. Catalysts make it easier for the reactions to go to the products side, it makes it go a lot faster. Some reactants don't have enough energy to make it over the uncatalyzed reaction pathway, so when a catalyst makes a new reaction pathway more reactants go to the products side, with out all the hassle.
Catalysts help a lot when it comes to industry, 80% of processes in the chemical industry use catalysts. Because it helps make more products faster. One type of product used in the industry is Acetic Acid. Acetic acid is one of the world's most important intermediate chemical, and it's used in the manufacture of vinyl acetate monomer, purified terephthalic acid, acetic anhydride, and much more.
The largest end use for acetic acid is as a raw material for the production of vinyl acetate monomer.
Polyvinyl acetate and copolymers of VAM are used in the manufacture of paints, adhesives, paper coatings, textile treatments and plastics.
Acetic acid is used to produce purified terephthalic acid, which is a key intermediate for a range of applications, including polyester fibers, bottles for water and soft drinks, photographic film and magnetic tapes.
Another very important use for acetic acid is the production of acetic anhydride. Acetic anhydride has a wide range of applications, the predominant one being the production of cellulose acetate. Cellulose acetate is used to produce textile fibers and cigarette filter tow. Other applications for acetic anhydride are plastics, agricultural chemicals and pharmaceuticals.
Acetic acid is used to produce a broad range of acetate esters; the most important of which are ethyl acetate, n-butyl acetate and isopropyl acetate. These solvents find applications in coatings, inks,