Temp
E=1.8c+32 F to C
K=C+273 C to K
C=K-273 K to C
Oxidation and Reduction
+ 0 reduction, 0 + oxidation
0 - reduction, - 0 oxidation
loss gain
Bonds
Ane Ene Yne
Single bond Double Triple
2n+2(H).C1H4 2n CnH2-2.C3H4
meth, eth, prop, but, pent,
hex, hept, oct, non, dec
Calculate the energy in jouled,j,needed to heat
175g of water from 55.0C to 95.0C given
that the specific heat of water is 4.184J/gk
Fornula:q=m X Sh X T
J= mass(grams) X Specific heat X Temp
29288j=175 X 4.184 X 40 (95-55)
-Satusated is single bond
-Unsatusated is double & triple
Iocic:nonmetal with metal
Polar:reflect (F-H/H-F)
Nonpolar covalent:2 nonmetals that reflect
(H-H/H-H)
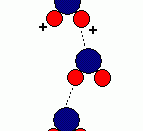
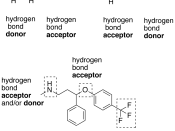
-to make a hydrogen bond it has to be
bonded to either or O,F,N
-soluble can they mix(dissolves)
likes dissolves like
polar dissolves polar/ionic
*when is says requires it means it's
endothermic,yes it affects
cause catalyst speeds up
and uses less energy