Investigate the rate of reaction between sodium thiosulphate and hydrochloric acid, when you change the concentration
Aim
The aim of this experiment is to investigate what affects the rate of reaction between Sodium Thiosulphate and Hydrochloric Acid. By using the variable of sodium thiosulphate concentration
Equations
Na2S2O3 + 2HCl = 2NaCl +SO2 H2O + S
Sodium thiosulphate + hydrochloric acid = Sodium Chloride + sulpur dioxide + water + sulpur
Background research
What is the most accurate way of obtaining the reaction rate between sodium thiosulphate and hydrochloric acid?
The average rate of a chemical reaction, over a certain time, is equal to the change in concentration of the reactant or the product divided by the time taken. Which method you use depends on the type of reaction it is. One way of finding this out is by carrying the reaction out in a thermostatically controlled bath - i.e.
one that you can control the temperature of. A sample of the reacting mixture is withdrawn with a pipette and the reaction within this bit of the mixture is stopped. This can be done in a number of ways like removing one of the reagents or suddenly cooling the mixture. You then perform a titration to find the concentration of one of the reactants or one of the products. If you do this at regular intervals, you can determine the rate at which the reaction is happening. In a reaction where a gas is formed, you have to use a different method. The volume of gas must be recorded at various times.
The rate of a reaction is defined as the speed at which the reaction takes place.
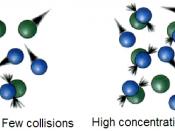
Most molecular collisions do not result in chemical change. Before any change takes place on collision, the colliding molecules must have a...