Chemical reactions take part in everyday life. Everything from decomposition to chemical reactions in our body, they all take place without our help. In a chemical reaction, some bonds are broken in the reactants in order to form bonds in the products, and atoms are rearranged. Chemical reactions always involve a change in energy. Energy is neither created nor destroyed; it is absorbed or released in chemical reactions. The reactions can be described as endothermic (energy is absorbed) or exothermic (energy is released). Signs that a chemical reaction is taking place can be (1) the substance has different properties than its reactants (2) a change in color (3) forming a gas or solid precipitate (4) or energy is transferred.
There are five general types of chemical reactions. The first is a synthesis reaction which two or more simple substances combine to form a more complex substance 2H2+O2 2H2O (reactant + reactant product).
The second is a decomposition reaction which is when a more complex substance breaks down into it's more simple parts 2H2O 2H2+O2 (reactant product + product). The third is a combustion reaction which at least one product of the reaction always contains oxygen CH4 + 2O2 CO2 + 2H2O (hydrocarbon + oxygen carbon dioxide + water). The fourth is single replacement reaction which is when a single uncombined element replaces another in a compound Zn + 2HCl ZnCl2 + H2 (reactant + reactant product + product). The fifth is double replacement reaction which is when parts of two compounds switch places to form two new compounds AgNO3 + NaCl AgCl + NaNO3 (reactant + reactant product + product).
Chemical equations use formuals to show the reactants and products in a chemical reaction. They must be balanced so that the number of atoms of each element on the right...
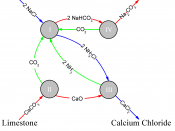
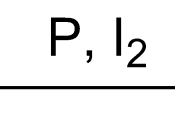
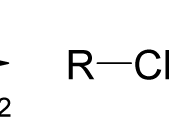
Chemical Reactions
I thought this was very informative - minus the symbols- :) try computers can surprise you. Each topic was also broken down and explained.
1 out of 1 people found this comment useful.