Chemistry Buffers: Bicarbonate buffers
- what a buffer is
- what a Bicarbonate buffer is and how it works
- What would happen if Bicarbonate Buffers didn't exsist.
Buffers ( I had example diagrams on my word program, but they didn't copy over. You can find these diagrams on the sites listed at the bottom.)
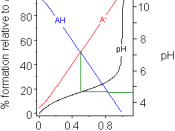
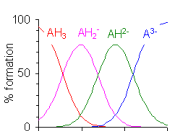
A Buffer is a mixture of an acid and its conjugate base, or a Base and its conjugate acid. A Buffer works by reacting with any new strong acid or base added to the mixture of the buffer. It reacts with the base or acid added so that the new base/acid wont change the pH of the mixture. The way buffer does this is by reacting with the strong acid/base to form a weak acid/base that will barley affect the pH if any effect.
Blood is a good example of how buffers work.
The pH of blood is around 7.4 and if it changes by only .2 it can be enough to kill a person. This is why your blood has buffers, because the buffers keep the bloods pH from changing and without them, we wouldn't be able to live. Blood contains 3 different buffer systems so that the blood will be able too buffer any kind of acid/base that enters it. Bicarbonate buffer is one of them and is a very important buffer. This is how a bicarbonate buffer works when too much base is added too the blood system:
As you can see the buffer has taken a highly basic material, and created two neutral products, that won't have much of an effect on the pH of the system.
This is how a bicarbonate buffer works when too much acid is put into a system:
As you can see in...