Full Lab Report
Experiment #2:
Acid-Base Titration
Lab Description: Acid-Base Titration
Introduction
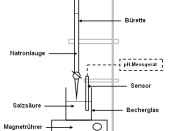
In this lab exercise we will evaluate the effectiveness of several indicators for the determination of the point of completion of a specific acid-base neutralization reaction. We will also determine the unknown concentration of the strong base NaOH by its reaction with a known amount of the weak acid, potassium acid phtalate (HKC8H4O4, abbreviated KHP). This will be accomplished using the titration method. The KHP solution will be created and its volume and concentration recorded. The KHP solution will be poured in a flask along with a few drops of one of three indicators we will be evaluating. The NaOH solution will be poured into a buret (with volume markers) and will be used as the titrant. The strong base will be added slowly to the acidic solution, gradually neutralizing the acid. The volume of base added can be determined by the difference in the initial and final volume marks on the buret.
At a certain volume of added NaOH, all the KHP acid will be neutralized due to the large equilibrium dissociation constant (Kb) of the base. This point of titration is referred to as the equivalence point. Considering the 1:1 stoichiometry of this acid-base reaction
NaOH(aq) + C6H4(COOH)(COOK) (aq) C6H4(COONa)(COOK)(aq) + H2O(l)
the point of equivalence is the point of titration when the number of moles of NaOH (Na) added is equal to the number of moles of KHP (Nb) in the solution. The number of moles of KHP in the solution can be calculated very simply by dividing the known mass of the sample in the solution by its molecular mass. The unknown concentration of the NaOH can then be calculated in the following manner:
At the point of equivalence of a reaction of 1:1...