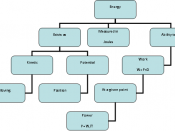
Energy- the ability to do work or produce Heat
*Potential
*Kinetic
Potential Energy- Energy that is stored; comes from composition or position of object
Ex: Energy stored in foods and fuels energy possessed by H2O behind a dam
Chemical Potential Energy- Stored Energy held within the bonds that hold atoms or molecules together. Some molecules hold more energy than others.
Ex: Fuels like gasoline
Ex: Foods like carbs
Kinetic Energy- Energy in motion. Kinetic energy is directly related to the constant, random motion of its atoms.
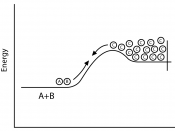
2 different types of energy reactions:
*Endothermic- reaction where energy is added in order for reaction to take place
*Exothermic- energy (heat) is given off after or during the reaction (explosions)
*Most of the time, you can actually feel the heat.
A + B Ã AB + (heat)
(Reactants) Ã (Products)
à = Chemical change
Law of Conservation of Energy- Energy can only change forms.
It cannot be destroyed or created.
Ex: Solar à Electrical Nuclear à Hydraulic Power
Law of Conservation of Mass- Matter cannot be created or destroyed. It can only change forms into other matter or energy.
YOU NEVER DESTROY MATTER!!
Ex: A + B Ã AB
40g + B Ã 70g
B Ã 30g
Phases of Matter
Evaporation- liquid to gas
Condensation- gas to liquid
Sublimation- solid to gas
Precipitation- gas to liquid
Freezing- liquid to solid
Melting- solid to liquid