Chlorofluorocarbons (CFCs) are a very present gas in our modern day world. Discovered by Thomas Midgley, an organic chemist at General Motors Corporation in 1920. He was looking for inert, non-toxic, non-flammable compounds with low boiling points that could be used as a method of cooling. Chlorofluorocarbons are now essential tools needed for various house hold utilities, they can be found in air conditioners, refrigerators, spray cans and other common items. Yet they have devastating effects on the environment, the reduction of chlorofluorocarbons is crucial in preventing further destruction to out Ozone layer, health and agriculture. Several alternatives are in development but must be implemented faster into our society.
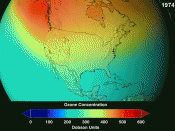
During the mid 1970's scientists discovered that chlorofluorocarbons where a major cause of ozone depletion in the upper atmosphere. Because chlorofluorocarbons are relatively inert they are able to travel to our upper atmosphere where they slowly diffuse and are broken down by the suns ultraviolet rays causing the highly reactive chlorine atoms within the chlorofluorocarbons to dissociate and move around freely.
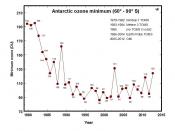
These chlorine atoms react with the earth atmosphere in a destructive fashion. Studies have shown that one chlorine atom destroys an average of 100,000 ozone molecules(Landstone 1). During the summer of 1985 60% of the earth ozone layer had been destroyed. This destruction of the ozone layer has ultimately caused a hole to develop over Antarctica. Causing devastating effects on the environment and human health. Since chlorofluorocarbons molecules have the ability to destroy the ozone layer at such an alarming rate, the United States has banned the use of chlorofluorocarbons for industrial processes. Instead, a number of substitutes for chlorofluorocarbons have been introduced, such as hydrochlorofluorocarbons (HCFCs). Since hydrochlorofluorocarbons (ARIC 1) are much more reactive than chlorofluorocarbons, when they are emitted into the atmosphere they break down in the troposphere...

A lot of information
You bring up a good topic, CFC's are just too damaging to the ozone layer. I agree with you, we really should replace CFCs.
1 out of 1 people found this comment useful.