No other half-century in history has witnessed so revolutionary a transformation in man's view of the nature of the physical universe as the one through which we have just passed. We have seen not only a vast accumulation of new facts but a profound revision of basic ideas and principles.
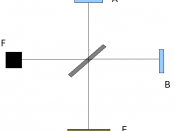
In its simplest form, quantum physics is the study of matter and radiation at an atomic level, where things work very differently from "our" world. A good example of this difference can be seen the the two broad types of phenomena that we are used to: particles and waves. Particles exist in one place at a time. Waves, such as sound waves, are spread out in space. At the atomic level, this distinction does not apply. Electrons, which are thought of as particles, can behave like waves. Similarly, in the case of light, which used to be thought of as occurring in waves, some behavior can only be explained if light exists in the form of particles, known as photons.
This wave-particle double-nature or "duality" can only be accounted for by quantum physics.
Classical physics can be mainly regarded as starting from about 1665. At the age of 23, Issac Newton enunciated the principles of mechanics, formulated the law of universal gravitation, separated white light into colors, proposed a theory for the propagation of light, and invented differential and integral calculus. Newton's contributions covered an enormous range of natural phenomena: He was thus able to show that not only Kepler's laws of planetary motion but also Galileo's discoveries of falling bodies follow a combination of his own second law of motion and the law of gravitation, and to predict the appearance of comets, explain the effect of the moon in producing the tides, and explain the precession of the equinoxes.