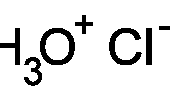Preliminary Investigation:
Aim:
To investigate the rate of reaction in different concentrations of acid to find the best suitable metal from a variety of metals.
Plan:
In the preliminary work I am going to investigate which metal out of 4 metals is the best for the investigation. These metals are Mg (Magnesium), Cu (Copper), Zn (Zinc) and Ca (Calcium). To do this i will investigate the reaction with different concentrations of hydrochloric acid. I will choose the best metal out of the 4 looking closely at its qualities e.g. the reaction times, reliability of results, metal properties etc. I will use this preliminary work to help me make my prediction; decide upon the most useful metal and to help clarify on how I will go about my experiment.
Apparatus:
- 3 Magnesium Strips
- 3 Copper Strips
- 3 Pieces of Zinc
- 3 Pieces of Calcium
- 12 Test Tubes
- 120ml of 0.25M,
0.50M, 1.0M, 2.0M and a 3.0m each of Hydrochloric acid (HCl).
- Measuring Cylinder
- Safety Goggles
- Stop Clock
- Scissors
- Ruler
Metals and Time each took to Stop Reacting in Seconds
Concentration
(Hydrochloric AcidMg
MagnesiumCu
CopperZn
ZincCa
Calcium
0.25M28.10X1380.0238.00
0.50M14.19X180.2836.00
1.00M1.38X60.5820.00
2.00M42.00X180.0628
3.00M36X960.3015.00
Results of Preliminary Investigation:
Preliminary Work Conclusion:
I conclude from the results above we can see that the metals had all very diverse results. From the above I noticed some properties which will help me decide which I will use. Zinc is not a very fitting metal because it can make the results very unreliable and inaccurate. It will not be a fair test as the metal comes in different sizes and weights, they are found in nuggets, and so each zinc piece cannot have the exact weight or length unlike magnesium thus making the experiment's results not...