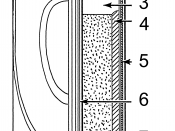
The dry cell or Leclanche cell was the first commercial battery and therefore had a big impact on society. It is the most common and the cheapest of the commercially available cell and is most widely used in torches, portable radios and battery-operated clocks. It is best used for low drain appliances, which need only small currents such as portable items (radio).
It consists of a zinc outer casting, the anode, an aqueous paste of ammonium chloride and a mixture of powdered carbon and manganese dioxide around a carbon rod, which is the cathode. Initially no zinc chloride is present, but as the cell is used zinc ions are formed and ammonium ions are discharged.
The reaction at the anode, the zinc cylinder is: Zn(s) â Zn2+(aq) + 2e-
At carbon rod (cathode), reduction half reaction is: MnO2(s)+ NH4+ + H2O(l) + e- â Mn(OH) 3 + NH3
This dry cell has a voltage of 1.5V
and is robust, easy to store and uses and causes minimal environmental problems upon disposal. The manganese (III) is readily oxidised to insoluble manganese (IV) oxide and so immobilised, the small quantities of zinc are not a problem and ammonium salts and carbon are harmless.
Disadvantages of the cell are that it does not contain a very large amount of electricity for its size and cannot develop very high currents. It can also develop leaks when it goes flat. It is non-rechargeable and has a short shelf life due to the acidic paste.
The mercury button cell produces a voltage of 1.3V, which remains fairly constant until the end of its life. Although it produces a lower voltage than the dry cell, the button cell can handle greater drain rates without a rapid drop in output voltage and has a longer shelf life. However,