* When water and methylated spirits are mixed together the molecules of each of the two liquids fit together more compactly than on their own. One example of this would be tessellation. Some shapes aren't able two tessellate on their own but with another shape they are able to.
* A molecule is made up of many atoms. And an atom is the smallest known particle there is other than electrons and protons.
* There are 3 different type of chemical bonds.
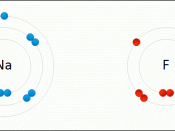
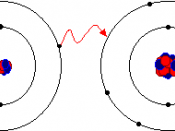
* Ionic bonds are formed by one atom transferring electrons to another atom to form ions. These bonds are between metals and non-metals. Potassium oxide is one example.
* Covalent bonds are formed by atoms sharing electrons to form molecules. This type of bond usually formed between two non-metallic elements. Examples of this is two chlorine atoms joining, or the atoms of 3 hydrogen atoms joining with an oxygen atom to form water.
* Metallic bonds are formed by two metal atoms. Metallic bonds are the strongest bonds of the 3.
* Matter cannot be created or destroyed in an isolated system.
* The 3 main pH indicators are litmus paper, universal indicator and phenolphthalein
* pH level vary from 1 to 14
* 1 is a strong acid
* 14 is a strong base
* 7 is neutral
* Orange juice and lemon juice are examples of acids.
* Bleach and sodium bicarbonate are both bases of around 9
* Salt Water is an example of a neutral product
* Acid + Base = Salt + Water
* Acid + Metal = Salt + Hydrogen
* Acid + Carbonate = Salt + Water + Carbon Dioxide
* Atomic mass is the sum of the number of protons and neutrons and is also shown on the top left...