Core 1 summary notes
Fossils fuels provide both energy & raw materials such as ethylene, for production of other substances
Identify the industrial source of ethylene from the cracking of some of the fractions from the refining of petroleum
Catalytic cracking is the process whereby high molecular weight fractions are broken down to low molecular weight ones. This process is used in petroleum refineries where crude oil is broken down to smaller alkenes and alkanes, until ethene, propene, (or both) are formed. Catalytic Cracking allows greater output of high-demand products.
Identify that ethylene, because of the high reactivity of its double bond, is readily transformed into many useful products
Ethylene, because of the high reactivity of its double bond, can form many useful products, such as plastics (polyethylene). For example, ethene can react with water to form ethanol, with a H3PO4 catalyst at 300oC. Ethene can react with oxygen in the presence of an Ag catalyst and at 250oC, to form ethylene oxide, which is further treated with dilute acid solution to form ethylene glycol.
Ethene can also react with oxygen, with a copper chloride catalyst and at 150oC, to form vinyl chloride (chloro-ethene)
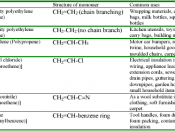
Identify that ethylene serves as a monomer from which polymers are made
Ethylene is the monomer which is converted into the polymer polyethylene by the process of polymerisation (chemical reaction where many small identical molecules join together to form one large molecule).
Identify data, plan and perform a first-hand investigation to compare the reactivities of appropriate alkenes with the corresponding alkanes in bromine water and iodine in solution
Alkanes burn in air to form CO2 and H2O, and liberate large amounts of heat in the process. Alkanes also react with (or decolourise) Cl, Br, and I, very slowly, in the presence of ultra-violet light. However without...

This stuff is superb
an EXCELLENT summary of the production of materials topic :)
great as notes.
0 out of 0 people found this comment useful.