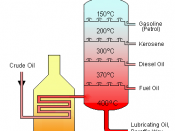
Petroleum products are used everyday. They are everywhere. In fact, you are probably using one right now. Although everyone uses petroleum, not many people know much about it. In crude oils raw state, it is useless. You can't use it in your car or in a plane or furnace. You have to use a process called fractional distillation.
Crude oil is actually many different substances combined together. Together, they are worthless. But separate, they are very useful. Refineries reassemble the hydrocarbon components of crude oil to develop products such as diesel fuel, jet fuel, and gasoline. When it first arrives at the factory, the crude oil undergoes fractional distillation. All of the different compounds in the oil have different boiling points and can be separated by this method. The oil is heated to a certain decree, certain parts vaporize, rise and flow through tubes, condense, and settle.
The lighter hydrocarbons are used for furnaces, gasoline, or stove oil. Some light distillates are naphtha and kerosene. Naphtha is used for gasoline and kerosene is used for jet fuel and stove oil. The middle distillates are either made into jet oil or further broken down into gasoline. The last group is the residual products, which could be severely processed into gasoline but is usually used for surfacing roadways and roofs.
Crude oil is formed over millions of years. In fact, the reason that they are called fossil fuels is because they formed in the same geological time frame as fossils, many years ago. The basic way that crude oil is produced is the same for all of the different scenarios. The first is called peat. Peat is the first stage of fuel formation and is used for burning. Many people, to this day, still use dried peat as fuel. Lignite,