The Nitrogen Cycle
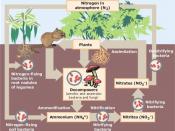
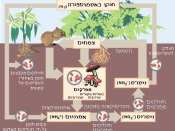
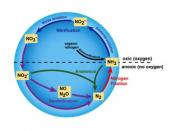
The nitrogen cycle represents one of the most important nutrient cycles found in terrestrial ecosystems (Figure 1). Nitrogen is used by living organisms to produce a number of complex organic molecules like amino acids, proteins, and nucleic acids. The largest store of nitrogen is found in the atmosphere where it exists as a gas (mainly N2). The atmospheric store is about one million times larger than the total nitrogen contained in living organisms. Other major stores of nitrogen include organic matter in soil and the oceans. Despite its abundance in the atmosphere, nitrogen is often the most limiting nutrient for plant growth. This problem occurs because most plants can only take up nitrogen in two solid forms: ammonium ion (NH4+ ) and the ion nitrate (NO3- ). Most plants obtain the nitrogen they need as inorganic nitrate from the soil solution. Ammonium is used less by plants for uptake because in large concentrations it is extremely toxic.
Animals receive the required nitrogen they need for metabolism, growth, and reproduction by the consumption of living or dead organic matter containing molecules composed partially of nitrogen.
In most ecosystems nitrogen is primarily stored in living and dead organic matter. This organic nitrogen is converted into inorganic forms when it re-enters the biogeochemical cycle via decomposition. Decomposers, found in the upper soil layer, chemically modify the nitrogen found in organic matter from ammonia (NH3 ) to ammonium salts (NH4+ ). This process is known as mineralization and it is carried out by a variety of bacteria, actinomycetes, and fungi.
Nitrogen in the form of ammonium can be absorbed onto the surfaces of clay particles in the soil. The ion of ammonium has a positive molecular charge is normally held by soil colloids. This process is sometimes called micelle fixation .Ammonium...