Ozone, a gas very similar to oxygen, can both help and harm humans, yet a problem has recently risen as levels of "good" ozone is being depleted. However, the source of this depletion created a big controversy when it was discovered, and, although it is now rarely mentioned, a dispute still exists. The problem arises that not all scientists, and other people for that matter, believe that chlorofluorocarbons (CFC's) are enhancing the problem, which is the commonly accepted theory. Yet before the problem can be understood the composition of the ozone layer must be discussed.
While ozone is a gas made of the same atom as oxygen, it has a different number of these atoms, altering its physical characteristics slightly. For example, ozone has a very pungent smell and a slight blue color. The ozone layer is in the stratosphere, the second layer of the atmosphere, and is located approximately ten to forty kilometers (six to twenty-five miles) above the earth.
While there is only one chemical form of ozone, it can serve to both help the environment and hurt it.
"Bad" ozone is found close to the ground, in the air which humans and other life forms breathe, acting as a pollutant. Automobile exhausts and other chemicals can create this type of ozone. Another major problem, besides pollution, which the harmful ozone contributes to, is smog on roads, making it hard for drivers to see. When sunlight shines on ozone, it creates smog, which has a brownish-yellow color. This can cause people to cough and make eyes water, as well as damage lungs, which can eventually kill people who already have lung diseases. However, this ozone can be beneficial by absorbing ultraviolet rays that reach the earth.
Contrary to this, "good" ozone is found high in the...
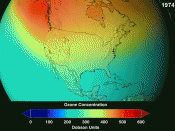

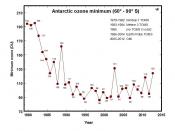
Ozone Layer...
The author did a good job of researching material, and obviously put time into writing this paper.
It uses "simple" language to describe a complex topic. Being easy to understand makes it a good resource for research.
Good Job.
4 out of 6 people found this comment useful.