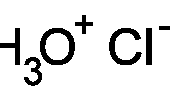Solutions provided
Sodium Thiosulphate 40g/l
Hydrochloric acid 73g/l
Planning
How will we time the reaction?
We will put the solution on top of the cross and add the chemicals together. At this point, start the stopwatch. The solution will begin to go cloudy. You should stop the watch when you can no longer see the cross.
What variables do we need to control?
We need to control the temperature, this should be room temperature. We need to make sure the volume of the acid is 10 cm3 and that the volume of Sodium Thiosulphate is 50 cm3. We will need to use the same cross every time and that we use 73g/l of concentrated hydrochloric Acid.
How will I change the concentration of Sodium Thiosulphate?
We will change the concentration by diluting it with water, we must always remember to keep the volume at 50 cm3. We need to do preliminary work to ensure that we get a good range of results.
We will use the value for the controlled variables we decided above.
Volume of Sodium Thiosulphate (cm3) Volume of water (cm3) Time for cross to disappear (s)
50.0 0.0 31.56
25.0 25.0 61.78
10.0 40.0 167.25
There is not enough range of different times between 0.0 and 50.0 or 25 and 25 to fit 5 different concentrations in. so we can get between 50.0 and 0.0 and 10.0 and 40.0 to get a descent set of concentrations.
Volume of Sodium Thiosulphate (cm3) Volume of water (cm3) Concentration (g/l)
50.0 0.0 40.0
40.0 10.0 32.0
30.0 20.0 24.0
20.0 30.0 16.0
10.0 40.0 8.0
How will I check my results?
I will check my results by doing each experiment three times.
How will I be safe?
I will obey the laboratory rules, as the experiment provides no particular...