Materials:1). 0.02 mol dm-3 Potassium Manganate2). 0.2 mol dm-3 Glucose3). 2 mol dm-3 Sulphuric Acid4). 50 cm3 Burette5). 250 cm3 Beaker6). Measuring Cylinder7). Thermometer (-10 to 110 oC)Hypothesis:By adding glucose to a solution of KMnO4 and H2SO4, the solution will turn colourless and it will take a specific time for the reaction to complete. By increasing the temperature, the time it takes (the rate) for the solution to completely change to colourless will be increased. Thus it can be said, that it is being hypothesized that an ÃÂincrease in temperature will cause an equal increase in the rate of the reactionÃÂ.
Method:1). Using a measuring cylinder, place 50 cm3 of sulphuric acid into a 250 cm3 beaker, add 50 cm3 of water using the same measuring cylinder, and then, from a burette, run in 5 cm3 of the potassium manganate (VII) solution. Heat the resulting mixture to about 55 oC, stirring gently while heating.
Place a beaker containing the hot solution on a white tile or a piece of paper.
2). Noting the time, pour 20 cm3 of the glucose solution (using a measuring cylinder) into the beaker. Swirl and then measure the temperature. Note the time when the solution turns colourless.
4). Record the results in table as seen on the proceeding page.
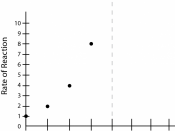
5). Plot a graph of time against temperature (since temperature is the independent variable).
6). Analyze the graph after the values have been plotted. Comment on the line of best fit and other deductions that can be seen.
7). Plot a graph of concentration of KMnO4 against time and deduce whether the reaction is zero order, first order or second order. Take the concentration as 0.02 mol dm-3.
Changing VariablesThe temperature is changed.
Constant VariablesThe amount of glucose solution remains the same.
The volumes of Potassium...