Electrolysis
In archaeological conservation, a cleaning process, especially of material from underwater archaeology, involving immersing the object in a chemical solution and passing a weak current between it and a surrounding metal grille. Corrosive salts move slowly from the object (cathode) to the grille (anode), leaving the artefact clean.
Electrolysis
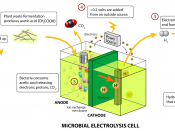
In chemistry, the production of chemical changes by passing an electric current through a solution or molten salt (the electrolyte), resulting in the migration of ions to the electrodes: positive ions (cations) to the negative electrode (cathode) and negative ions (anions) to the positive electrode (anode). During electrolysis, the ions react with the electrode, either receiving or giving up electrons. The resultant atoms may be liberated as a gas, or deposited as a solid on the electrode, in amounts that are proportional to the amount of current passed, as discovered by English chemist Michael Faraday. For instance, when acidified water is electrolysed, hydrogen ions (H+) at the cathode receive electrons to form hydrogen gas;
Electrolysis
Removal of unwanted hair using an electric current.
It can be very effective in experienced hands, but is slow and therefore expensive.