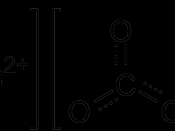
Describe the properties of acids with regard to their reactions with metals, bases and carbonates.
Acids taste sour, conducts electricity and has a pH lower than 7. Therefore, it turns red if universal indicator is added and turns blue litmus paper red. Strong acids with a pH of 1 or 2, contain more hydrogen ions and are very corrosive while weak acids with a pH of 5 or 6 contain less hydrogen ions therefore, they are not that corrosive.
When an acid is added with a metal, salt and hydrogen are produced. Hydrogen, which quickly bubbles off during the reaction is displaced by the metal added. The bubbles will stop when all the acid has been used up.
When an acid is added with a base, salt and water are produced.
When an acid is added with a carbonate, salt, water and carbon dioxide are produced.
2. Explain the test for carbonate ions using dilute hydrochloric acid and limewater.
Carbonate ions can be tested by adding dilute hydrochloric acid and putting the given off gas into limewater. If carbonate ions are present, limewater will turn milky or cloudy because when an acid and carbonate react, it gives of carbon dioxide, which turns limewater cloudy. For example:Sodium Carbonate + Hydrochloric acid â Sodium Chloride + Water + Carbon DioxideNa2CO3 + 2 HCl â 2 NaCl + H2O + CO2The collected carbon dioxide will therefore turn limewater cloudy. Limewater is a common name for saturated calcium hydroxide solution, Ca(OH)2. When carbon dioxide is added to limewater, water and calcium carbonate (chalk) are produced. In limewater, if carbon dioxide are present, a white precipitation of calcium carbonate (chalk) will form.
Limewater + Carbon dioxide â Calcium Carbonate + WaterCa(OH)2 + CO2 â CaCO3 + H2O3. Describe how solutions are acidic, alkaline or neutral...