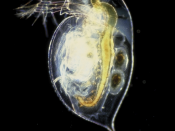
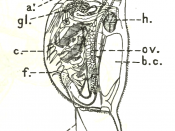
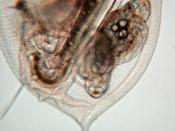
Daphnia are small animals. They are plankton to be precise and they are a major component in the food chain in freshwater environments such as lakes, ponds, rivers, swamps and even in some acidic swamps. Mostly fishes and amphibians such as; toads and frogs feed on them. Hence their rate of growth and reproduction is of a high importance for the reproduction and survival rate of the above mentioned species. In terms of their biological taxonomic unit they belong to the kingdom Animalia, phylum Arthropoda, subphylum Crustacea, class Branchiopoda, order Cladocera, family Daphniidae and genus Daphnia.
Daphnia reproduce sexually and parthenogenetically (Sterner, 1998). Parthenogenesis is the asexual reproduction of a species from females only. The females reproduce more females from their unfertilized eggs. No males are available in a parthenogenetic reproduction. Men are only produced for sexual fertilization only, which will help to introduce more variability in the gene pool of the species via sexual reproduction.
After the males fertilize the eggs, they die. Sexual reproduction is performed in mid autumn were the conditions for such reproduction is the best (Sterner, 1998). The peak season of DaphniaÃÂs asexual/ parthenogenetical reproduction starts from mid spring to late summer (Sterner, 1998). The sexual fraction of DaphniaÃÂs population ranges between a minimum of 3.0% to a maximum 8.0% of the total population all year around. Hence, the species is considered to be a parthenogenetic species (Hülsmann and Weiler 2000).
The rate of survival and the rate of reproduction of Daphnia are highly influenced by the fluctuations and changes that occur in the environmental conditions of their surrounding habitats (Hülsmann and Weiler 2000). Three of the most influential environmental conditions that easily alter these rates are; ÃÂlake-origin and food concentrationÃÂ, ÃÂpredation by other organismsàand ÃÂtemperatureà(Hülsmann and Weiler 2000).
Out of the three factors mentioned above, ÃÂpredation by other organismsÃÂ has the lowest effect on the rate of survival and the rate or reproduction of Daphnia as concluded by the results of the experiment that was conducted by Sterner (Sterner, 1998). The most important factor is ÃÂlake-origin and food concentrationÃÂ followed by the second relevant factor, namely the change in the ÃÂtemperatureÃÂ of the environment of Daphnia (Sterner, 1998). Daphnia are mostly filter feeder that feed on algae, small organic particles and smaller organisms like rotifers and bacteria. Their food supply is directly proportional to the ÃÂprimary productionÃÂ of the aquatic environment that they are living in (Sterner, 1998). In order to determine the rates of survival and reproduction of Daphnia under different food concentrations, a ÃÂstarvation-experimentÃÂ was conducted on two different taxons of Daphnia; Daphnia Hyaline and Daphnia Galeata (Rellstab and Spaak 2009). Also hybrids of these two Daphnia were used in the experiment. Both of these Daphnia were obtained from three different lakes. The first lake is Lake-Brienz (BRZ) that is located in northern parts of the Swiss Alps. A cold and oligotrophic lake that conducts low levels of primary production. Hence it has low levels of algae and the water is very clear and rich in oxygen (Rellstab and Spaak 2009). The second lake was Lake-Constance (CON) which is between the borders of Switzerland and Germany. This lake is a mesotrophic lake which conducts mid-level of primary production and is inhibited by low to mid levels of algae and rotifers. A mesotrophic lake offers more food than needed to the Daphnia (Rellstab and Spaak 2009). The third lake is Lake-Greifensee (GRE) situated in low Swiss land. This lake is eutrophic and conducts high levels of primary production. Algae are abundant in the lake as well as many fish species and other amphibians (Rellstab and Spaak 2009).
The starvation-experiment was conducted under laboratory conditions which allowed the scientists to have control over temperature and food concentration of the living environments of the Daphnia. 170 neonates (newborns) of the two taxons mentioned above were randomly selected from all of the three different lakes listed above. The largest neonates among those randomly selected animals were the neonates originating from CON. Each neonate was placed in a 100 mL jar. The jars were filled with filtered water obtained from a fourth oligotroph Lace-Lucerne so that no Daphnia has the advantage of survival over the other one by being exposed to its domestic water. The jars were maintained at 5úC to simulate harsh winter conditions. Two food conditions were chosen for this experiment; a low-food condition and a no-food condition. If the neonates were reproducing their offspring were separated and put in a no-food environment (Rellstab and Spaak 2009). Three major responses were targeted in this experiment; the ÃÂlife spanÃÂ, the ÃÂreproduction rate and population growthàand the ÃÂlife span and reproduction of the offspringàof Daphnia under these two different food conditions (Rellstab and Spaak 2009).
Under the no-food condition the neonates from BRZ and GRE had the highest and the fastest mortality rates. Most of the neonates of these two lakes started to die after 4 days and all of them died within 13 to 22 days under both food conditions. However, the longest living neonates of CON survived up to 36 days and 126 days in no-food and low-food conditions respectively. An expected behavior was the sinusoidal rate of mortality which increased and decreased over and over as observed by Stephan Hülsmann and Winfried Weiler (Hülsmann and Weiler 2000). It turned out that this is a result of ÃÂnatural-selectionÃÂ. The fittest survive longer than the weaker ones until they can not resist the harsh conditions anymore. As far as the ÃÂreproduction rate and population growthàit was observed that none of the animals that were kept under no-food conditions were able to produce any offspring. All of the animals from all three different lakes were able to produce offspring, however with a total of 14 offspring the animals from CON had the highest number of offspring compared to 6 and 3 offspring of Daphnia from GRE and BRZ respectively. The rate of population growth was positive only for the individuals that originated from CON, the other two representatives experienced negative population growth with respect to their mortality rates. The 23 total juvenile offspring were transferred to a no-food environment. The longest lifespan of the 2nd generation neonates was 25 days where as the shortest lifespan was 2 days. The 2nd generation neonates of CON lived the longest, followed by the neonates from GRE and BRZ respectively (Rellstab and Spaak 2009).
Based on these results the scientists concluded that Daphnia can survive harsh winter and high-oligotrophic lake conditions by parthenogenetic reproduction. Except for the case of the Daphnia originating from CON, the lake-origin did not influence the survival rate of the animals. In contrast to lake-origin, significant correspondence was detected between the life-span of the mother and the life-span of the neonate. Mothers from CON were the largest animals that were randomly selected. They survived for the longest period of time and gave birth to larger neonates compared to the rest of the 2nd generation neonates. The larger 2nd generation neonates did survive for the longest period of time as well. Hence, the scientist concluded that the body mass of the Daphnia can be used as a source of energy under harsh conditions. Further analysis of the results indicated that temperature is playing a key role in the survival and the reproduction of Daphnia as well. Other similar experiments that were conducted on Daphnia were undertaken at higher temperatures which range from 10 úC to 20 úC. In most cases higher temperature conditions were only beneficial during sexual production of Daphnia. Higher temperatures reduced the amount of time that is needed for the development of the sexually produced eggs (Hülsmann and Weiler 2000). However, the results of Rellstab and Spaak who conducted their experiment at 5 úC concluded that sexual reproduction requires more energy as opposed to asexual reproduction. Hence, at low temperatures Daphnia are compelled to reproduce asexually. The last conclusion made by these scientists is that Daphnia that inhabit mesotrophic lakes are the most successful animals compared with their other counterparts (Rellstab and Spaak 2009).
Based on the dedicated work and research activities of various scientists in the world, it can be concluded that the rate of lifespan and reproduction of Daphnia is highly related to their aquatic environment. Factors like food-concentration and temperature are the most important factors that can have the greatest impacts on the rate of survival and reproduction of Daphnia.
References:Hülsmann, S. and Weiler, W. (2000) Adult, not juvenile mortality as a major reason forthe midsummer decline of a Daphnia population. Oxford- Plankton Research, 22, 151-168.
Porter, K., Saunders, P. and Taylor, B. (1999) Population dynamics of Daphnia spp. andimplications for trophic interactions in small monomictic lake. Oxford- Plankton Research, 21, 1823-1845.
Rellstab, C. and Spaak, P. (2009) Lake Origin determines Daphnia population growthunder winter conditions. Oxford- Plankton Research, 31, 261-271.
Sterner, R. W. (1998) Demography of a natural population of Daphnia retrocurva in alake with low food quality. Oxford- Plankton Research, 20, 471-489.
Trabeau, M. et al. (2004) Midsummer decline of a Daphnia population attributed in partto cyanobacterial capsule production. Oxford- Plankton Research, 26, 949-961.