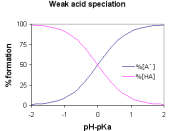
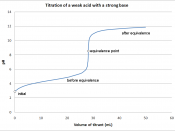
Half Equivalence points: Weak Acid = 10.3875 mL Strong Acid = 12.325 mL Comparison of two curves: The two curves have some major differences. First of all, the weak acid curve's lowest point is at about 4 while the lowest point on the strong acid curve is about 2. Also, the weak acid curve begins its drastic climb at about 20 mL while the strong acid curve begins its drastic climb at about 25 mL. One thing that the two graphs have in common is that they both have a high point of about 12.
The strong acid has a lower starting point because it has a lower pH. Also, the drastic rise of the strong acid curve starts later because it takes more of the base, NaOH, to raise the pH of the strong acid. Both curves flatten out at about the same point because the same base is being added to both acids.
Therefore, the final pH should be about the same for both graphs. Finally, the strong acid graph raises about 10 points of pH while the weak acid graph only raises about 8 points of pH.
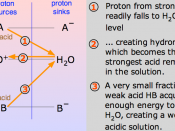
I still cant find the equation for Ka for the weak acid. I looked in the book but still couldn't find it. I emailed Jake and asked him to send it to me. If he does ill send it to you, if not ill talk to you about it in class on Wednesday.
If you think there is anything wrong with the paragraphs, feel free to change them.