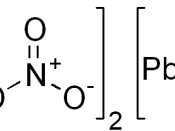
Aim The aim of the experiment was to investigate the effects of heating Lead Nitrate (Pb(NO3)2.
Method About three spatulas full of Lead Nitrate was poured into a small test tube. The Bunsen burner was lit in the usual way and set to the hottest flame. Using a test tube holder the test tube was carefully positioned about five centimetres above the bases of the flame. The experiment was halted prematurely because a crack appeared around the base of the test-tube. Also, every minute or so a glowing spindle was inserted into the test-tube to test for the presence of oxygen.
Safety Precautions Goggle were worn before and during the heating. Care was taken not to point the mouth of the test-tube towards anybody. Also lab coats were worn. When not in use the Bunsen burner was turned onto the yellow flame.
Observations It was noticed that a brown gas with a pungent smell was emitted from the test tube.
This was Nitrogen Dioxide. Another gas given of was oxygen. A small degree of de-crapitation was also noticed. The colour also changed to a shade of yellow. The residue was lead oxide.
Conclusion It was concluded that if Lead nitrate was heated an irreversible chemical change occurred.
Diagram