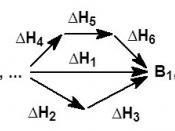
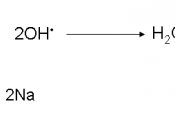
I was given the duty of determining whether or not the worker's story of being burnt is supported by scientific evidence. By using calorimetry and Hess's Law, the validity of the worker's claim can be found. The scientist's experiment was compared and evaluated with a similar one that was accomplished with less substance for safety reasons. 5 grams of NaOH was mixed with 41 ml of HCL. With this reaction, the H can be found by using the equation q=mc T. It was found that q=70759.04. From this result, H can be found by dividing q by the number of moles of NaOH. The results showed that H=70246.29. According to Hess's Law, it doesn't matter what pathway is used to make a substance because the H will always be the same. By comparing the worker's results with our own, the solution can be found. The equation used is 70759.04=(505)(4.18)(Tf-25).
With this equation it was determined that the final temperature of the scientists mixture was 60 degrees Celsius. Thus, the mixture was hot enough to shatter glass and burn the worker.