Purpose:
In this experiment a process for obtaining a substantially pure enantiomer of ibuprofen is described by the process that utilizes first an enantiomerically enriched mixture of ibuprofen. This enriched mixture dissolves in a solvent and solid racemic ibuprofen is separated, leaving a mother liquor comprising, the solvent and the enriched enantiomer substantially free of the other enantiomer. Racemic is an optically inactive because the two isomers rotate plane polarized light in opposite directions. In contrast to the two separate enantiomers, which generally have identical physical properties, a racemate often has different properties compared to either one of the pure enantiomers. Different melting points and solubility's are very common.
Reagent Table:
M.WM.PB.P DensitySolubility
Ibuprofen206.376immiscible
2-Propanol60.10-8682.3.785miscible
MTBE88.15-10955.2.74044.8
H2SO498.08103371.84miscible
Methyl benzyl Amine121.181185.939immiscible
Structures:
Ibuprofen
2-Propanol
MTBE
H2SO4
Methylbenzyl Amine
Observations and Data:
Part B
Weight of watch glass--- 28.563 grams
Weight of crystals and watch glass--- 31.047 grams
Weight of crystals--- 2.484
grams
Part C
Weight of (+)-Ibuprofen---54.226 grams
Weight of (+)-Ibuprofen Dry --- 54.545 grams
Weight of (+)-Ibuprofen---.319 grams
Class average ---1.992 grams
Angle of rotation --- 2.8 degrees
Part D
Weight of pre-weighted beaker with boiling chip--54.090 grams
Weight of product and beaker--54.744 grams
Weight of product--- .654 grams
Class average - 3.068 grams
Melting point of purity---162 degrees
Melting of impurity ---- 56 degrees
Discussion:
In this experiment a process for obtaining a substantially pure enantiomer of ibuprofen is described by the process that utilizes first an enantiomerically enriched mixture of ibuprofen. In chemistry racemization refers to partial conversion of one enantiomer into another. Chiral molecules have two forms which differ in their optical characteristics: The levorotatory form (âÂÂ) will rotate the plane of polarization of a beam of light to the left, while the dextrorotatory form (+) will rotate the plane of polarization of a beam of light...

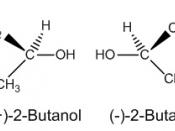

Ibuprofen Resolution
While the chemistry in this essay may be exceptional, the presentation as an essay is comparatively poor. I will point out just one example: there are two mistakes in the title: "Ibuprofen" should be capitalized, and the other word should be spelled properly: "Resolution." This is hardly A+ work.
0 out of 0 people found this comment useful.