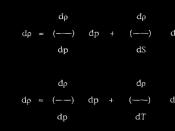The purpose is to determine the value of the ideal gas constant, RObservations:At first a few seconds after we flipped the tube upside down bubbles gradually formed faster and faster as the HCl react with Mg ribbon, soon about 1/3 of the tube was full of H2 , and after just about one minute the reaction had stopped and the tube was a little over half full. After that we let it cool off to room pressure for 4 more minutes.
Data Table For Ideal Gas Constant LabTrial 1Trial 2Mass of 2 meter of Mg ribbon (g/m)1.941.94Length of Mg ribbon (cm)4.404.40Mass of Mg ribbon (g)0.042680.04268Volume of gas (mL)44.5044.05Room temperature (oC)23.523.5Barometric Pressure (mm Hg)765.2765.2Calculations: Trial 1:Mass of Mg ribbon:1.94/200 =0.0097g/cm0.0097(4.4) =0.04268gVolume of H2 gas in liters:44.50mL of H2 gas = 0.0445LRoom temperature in K:0oC = 273.15K23.5oC = 296.65KPressure in atm:760mm Mg = 1 atm765.2mm Mg = 1.00684 atmCalculations: Trial 2:Mass of Mg ribbon:1.94/200
=0.0097g/cm0.0097(4.4) =0.04268gVolume of H2 gas in liters:44.05mL of H2 gas = 0.04405LRoom temperature in K:0oC = 273.15K23.5oC = 296.65KPressure in atm:760mm Mg = 1 atm765.2mm Mg = 1.00684 atmQuestions:1) Find the Pressure of hydrogen:Room temperature:23.5oCPressure of the water vapor: 21.7 mm HgPressure of H2: 765.2mm Hg ÃÂ 21.7 mm Hg = 743.5 mm Hg2) Balanced Equation:Mg (s) + 2HCl (aq) H2 (g) + MgCl2 (aq)3) Moles of Mg used:Molar Mass of Mg is 24.3g/mol0.04268/24.3 = 0.0017564 mol4) Moles of H2 produced:Same as moles of Mg used is 0.001756 mol5) Calculate R(P)(V)=(N)(R)(T)(1.00684)( 0.0445) = (0.001756)(R)( 296.65)0.0448 = .521R0.0860 (L)(atm)/(mol)(K) = RError Analysis:Percent Error:.0860- .0821 =.0039.0039/.0821 = .0475.0475(100) = 4.75%This % error is good because we are within 5%The most important error happens whenever we try to cover the hole in the rubber stopper with our finger. Some of the liquid leaked out through our finger. This had an effect on our result because when the liquid leaked out, air gets in, which will mix with H2, and increase the volume. It will then affect the answer latter on when we multiply the volume of H2 and the pressure. This error is significant because there could be 0.5 to 1 mL Increase of Liquid.
When we measure the length of Mg ribbon with a ruler, it might be off by .01cm because the smallest unit on the ruler is a mm. This will vary our result because we could have + or -.01cm. when we do the experiment it will effect how much of H2 we get once finished, because we have excise HCl and the limit reactant is Mg. This will effect the moles of Mg we got, then this will effect the moles of H2 . This error is significant because .01cm is large enough to make a difference in the final answer.
Finally after the reaction, we waited for 5 minutes for the tube to reach room temperature. Im not sure if 5 minutes is enough. If the Temperature is higher than room temperature then the gass takes up a larger volume increasing our answer. The error is sort of significant because we donÃÂt know exactly what the temperature is in the tube.
Conclusion:In conclusion I worked out the value of the ideal gas constant, (R) which is (0.0821)(L)(atm)/(mol)(K), but my final answer is (0.0860)(L)(atm)/(mol)(K). I had 4.75% of error, which isn't that big. We could improve this experiment by getting a better technique in covering the rubber stopper when we take the tube out of the liquid, or a more precise rulerp.s there is no bibliography because this was a class experiment