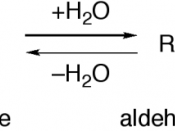
Identify the following organic compounds:
1: Hydrocarbons.
Hydrocarbons are chemical compounds of hydrogen and carbon; also called an organic compound. Hydrogen and carbon atoms can be combined in virtually countless ways to make a diversity of products. Carbon atoms form the skeleton of the hydrocarbon molecule, and may be arranged in chains (aliphatic) or rings (cyclic). There are three principal types of hydrocarbons that occur naturally in petroleum: paraffins, naphthenes, and aromatics, each with distinctive properties. Paraffins are aliphatic, the others cyclic. Paraffins and naphthenes are saturated; that is, they have a full complement of hydrogen atoms and, thus, only single bonds between carbon atoms. Aromatics are unsaturated, and have as part of their molecular structure at least one benzene ring, i.e., six carbon atoms in a ring configuration with alternating single and double bonds. Because of these double bonds, aromatics are usually more reactive than paraffins and naphthenes, and are thus prime starting materials for chemical synthesis.
Other types of hydrocarbons are formed during the petroleum refining process. Important among these are olefins and acetylenes. Olefins are unsaturated hydrocarbons with at least one double bond in the molecular structure, which may be in either an open chain or ring configuration; olefins are highly reactive. Acetylenes are also unsaturated and contain at least one triple bond in the molecule.
Saturated Hydrocarbons:
The number of hydrogen atoms associated with a given skeleton of carbon atoms may vary. When the chain or ring carries the full complement of hydrogen atoms, the hydrocarbon is said to be "saturated". Such hydrocarbons are known as paraffins, paraffinic hydrocarbons, or alkanes/cycloalkanes. The "paraffin" term was common in the oil industry, because of the waxy characteristics of crude which contains these molecules. The term "alkanes" is common among chemists.
hydrocarbon with the basic formula CnH2n+2; it...