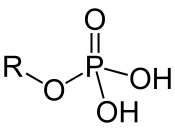
Infrared spectroscopy
Infrared spectroscopy is widely used to identify functional groups present in unknown organic substances. This is because particular bonds in organic molecules undergo vibrational excitation when they absorb infrared radiation. (1).
In compound 1, the major functional groups present include an alcohol and an alkene. The alcohol can be determined by the presence of an O-H stretch at about 3300 cm-1 and a C-O stretch at about 1050 cm-1. The alkene functional group gives a characteristic C=C stretch at about 1650 cm-1. This group is corroborated by the presence of a weak =C-H stretch at about 3050 cm-1 and a =C-H bend at about 950 cm-1. There are also alkyl groups present, as determined by the presence of a C-H stretch between 2850 and 2950 cm-1 and C-H bending between 1300 and 1500 cm-1. Given the three possible identities, compound 1 must be 4-penten-1-ol. (1)(2)
In compound 2, the major functional group present is an aldehyde.
This can be determined by the presence of a peak at about 2710 cm-1 and a peak at about 1715 cm-1. These two peaks corroborate each other. Alkyl groups are also present, based on the C-H stretch that appears from 2850-2950 cm-1 and the C-H bending that shows up between 1300 and 1500 cm-1. The spectrum of compound 2 fits best with 3-methylbutanal.
Compound 3 contains a ketone functional group. This can be determined by the presence of a C=O stretch at about 1710 cm-1 and the absence of any peak at 2700-2800 cm-1. An alkyl C-H stetch appears at 2850-2950 cm-1 and alkyl C-H bending shows up between 1300 and 1500 cm-1. From interpretation of the IR, compound 3 must be 2-pentanone.
Many of us are probably familiar with infrared radiation. Infrared radiation could be related with heat. Infrared is a...