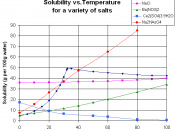
How does the solubility of Potassium Chloride (KCl) and Potassium Iodide (KI) in water vary with temperature?
Aim
To observe solubilities of KCl and KI with water at different temperatures
To compare the two solubility curves and discuss what might vary the solubility of different ionic compounds.
The variables
Dependent variable | Temperature |
Independent variable | Amount of solute (KCl, KI) |
Constants | Amount of the solvent (water), pressure |
Apparatus
100g of Potassium Chloride
100g of Potassium Iodide
10ml cylinder
Test tubes x 15
Stoppers
Glass rod
Beakers x 5
Distilled water
Spatula
Hot plate
Ice - to cool down the water
Thermometer (0ðC to 100ðC) x 2
Method
In this experiment different amount of solute were combined with 10ml of water (solvent) which originally was at 0ðC. The solvent and solute were mixed together and the temperature where the solute is completely dissolved to the solvent is measured.
Weigh different amounts of KCl and KI
KCl - 6g, 7g, 8g, 9g, 10g (approx.) |
KI - 12g, 14g, 16g, 20g, 25g (approx.) |
Measure 10ml of water and place them into test tubes
Place the test tubes into ice and wait till they cool to 0ðC
Start dissolving the substances into cooled distilled water with the smallest amount of substances
Use a glass rod to stir or a stopper to shake
Measure the temperature at where the substances are covmpletely dissolved
Repeat the process for each gram of the substances - use hot plates with water bath on top where appropriate to get higher temperature.
Data collection and processing
Potassium Chloride
When Potassium Chloride is completely dissolved in water, it becomes clear solution without any solid left in the solution.
Trial 1
Amount of KCl | Temperature that KCl is dissolved | |
5.909 g ñ0.1mg | 7 ðC ñ0.1ðC | Amount of water = 10cm3ñ0.1 cm3 x 2... |