Investigating how surface area affects rates of reaction.
Background Info:
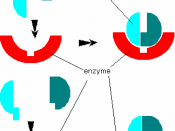
Enzymes are biological catalysts. They break down the chemical they are uniquely made for, such as proteins, fats and some harmful substances like Hydrogen Peroxide.
An enzyme basically works on a key and lock like system. The enzyme is unique to its self but many others may be close to the exact shape, but not quite. Therefore allowing substrates to fit into its activesite, which then breaks it gets broken down. As all enzymes are different some substrates will have to work harder to fit into the active site of another enzyme of a similar shape, as some will fit perfectly, they all have different shape activesites to deal with each of its designated chemicals.
The best conditions for enzymes to breakdown and live is around 37-40ðc /body temperature; in a low pH level are if would work best.
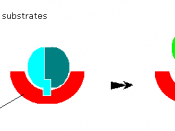
This will digest slowing as the substrates are not a good fit for
The active site.
Where as.
This fits perfectly so it will digest this fast and well.
When it's a perfect fit the two substrates join together and you get a product.
These processes have an equation.
Hydrogen Peroxide
2H2O2
How the surface area can affect reaction time.
Prediction:
My prediction for the experiment is that the smaller and more pieces you cut the potato into the faster the reaction will be, because more enzymes can work on it as the surface area is increased and they are smaller pieces so it should digest faster.
Equipment:
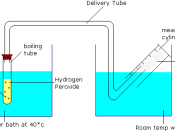
Cork border - To get a continuous even sized potato discs
Stopwatch - To time how long you've been running each test
Potato - To test with
20 vol. Hydrogen Peroxide - To place potato discs in to record reaction
Boiling tube - Place discs in while testing
Delivery tube - Take the oxygen into the other water bath
Measuring cylinder - To measure how much oxygen was produced
Hot water bath at around 40ðc - To place the boiling tube with discs in
Water bath - To collect the oxygen that's going into the measuring cylinder
Knife - To chop potato discs into 2mm in height
Ruler - To measure the 2mm
Diagram of setup equipment:
Fair test:
Run test for same length of time foe each added disc.
Vol. of Hydrogen Peroxide same
Same size potato discs
Same temperature water bath
Same length delivery tube
Formula:
For My Discs = 2 x ÃÂ x Radius2 + 2 x ÃÂ x Radius x Diameter
Surface area of one disc = 2 x ÃÂ x 2.5mm2 + 2 x 2.5 x 5mm
Method:
Use cork borer number 3 to get the potato cylinders.
Slice the cylinders into 2mm discs with knife, measure with ruler.
Put chronicle flask into beaker with just water about 80ðc and after 5 minutes swap for a new, as temperature will decrease.
Put a disc into the Hydrogen Peroxide for 10 minutes with the delivery tube connected and leading into the measuring cylinder.
Time with stopwatch 10 minutes and after that time, measure how much oxygen was produced from the reaction.
Repeat this 3 times to ensure accuracy and fairness then attain a average from the results.
Repeat all these steps with 20-10 discs.
Results Tables:
Volume of Oxygen Released In 10mins /ml
No.# Discs | Volume of Oxygen released in 10mins /ml | |||||
Surface Area /cm | T1 | T2 | T3 | Average | Rate Av /10 | |
1 | 1.2 | 7 | 7 | 0.7 | ||
2 | 2.4 | 9 | 9 | 0.9 | ||
3 | 3.6 | 11 | 11 | 1.1 | ||
4 | 4.8 | 13 | 13 | 1.3 | ||
5 | 6 | 15 | 15 | 1.5 | ||
6 | 7.2 | 17 | 17 | 1.7 | ||
7 | 8.4 | 230 | 20 | 2 | ||
8 | 9.6 | 23 | 23 | 2.3 | ||
9 | 10.8 | 25 | 25 | 2.5 | ||
10 | 12 | 28 | 28 | 2.8 |
Rate of Reaction
No.# Discs | T1 | T2 | T3 | Average | Rate Average /10 |
1 | 5.6 | 5.6 | 5.6 | 5.6 | 0.56 |
2 | 9.2 | 9.2 | 9.2 | 9.2 | 0.92 |
3 | 14.3 | 14.3 | 14.3 | 14.3 | 1.43 |
4 | 12.9 | 12.9 | 12.9 | 12.9 | 1.29 |
5 | 20.1 | 20.1 | 20.1 | 20.1 | 2.01 |
6 | 23.2 | 23.2 | 23.2 | 23.2 | 2.32 |
7 | 26.5 | 26.5 | 26.5 | 26.5 | 2.65 |
8 | 29.5 | 29.5 | 29.5 | 29.5 | 2.95 |
9 | 32.1 | 32.1 | 32.1 | 32.1 | 3.21 |
10 | 34.5 | 34.5 | 34.5 | 34.5 | 3.45 |
Analysis:
It seemed from the information we collected it shows that the more discs you would add the slower the reaction time. Also the more discs of potato we added the more Oxygen (O2) it produced.
Evaluation:
Overall my prediction was half correct as I stated that the bigger the surface area the more enzymes could digest it which would speed up the digestion time, how ever the more discs we added the slower the reaction was, as the enzymes can only work to the best of their abilities and not work faster with the increased surface area.