The Enthalpy Change of the Decomposition of Calcium Carbonate
Introduction
Research Question:
What is the enthalpy change of the decomposition of calcium carbonate?
Background:
Enthalpy in chemistry can be thought of as the energy contained within the bonds, or the internal energy, but it is not heat and you can only measure changes in it. When bond bonds break in the reactants energy is given off, when bonds form, energy is absorbed. If the energy absorbed is less than the energy released, then the reaction is exothermic and the products are more stable than the reactants and vice versa. But, the enthalpy, or change in energy, of some reactions are too difficult to carry out in a standard laboratory due to toxins released or conditions that cannot be met in simple laboratory. Thus, that's where Hess's Law comes in as it states that if you go from the reactants to the products in one reaction or many intermediate steps, the overall energy change is still the same.
In this experiment, Hess's law will be utilized to calculate the decomposition of Calcium carbonate. Calcium carbonate is a fairly stable compound, thus, it must mean that more energy goes into this reaction than out to produce the desired results.
It is the enthalpy change of this reaction that this experiment was designed to find. Of course, another route must be used to achieve this, so HCl is added to CaCO3 and to CaO, and then, through some calculations and the equation
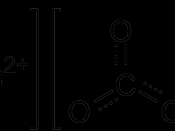
CaCO3 + 2HCl ( CaCl2 + CO2 + H2O
CaO + 2HCL( CaCl2 + H2O
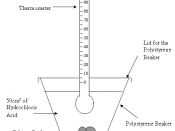
These two reactions will be done in a polystyrene beaker so no...