Title: Redox Reactions
Research Question: Is it possible to determine if a redox reaction took place by using only the equation?
Hypothesis: Yes, I believe it is possible to determine if this reaction took place by using the oxidation numbers in the equation.
Variables:
1. I cleaned the reaction surface to limit contamination
2. I held the chemicals at approximately a 90 degree angle for consistency
Introduction:
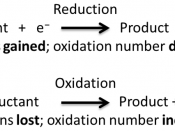
Redox reactions occur when both oxidation and reduction take place. All atoms can be assigned an oxidation number, which is a positive or negative number that help in determining the reducing and/or oxidizing agents in an equation. An increase in the oxidation number of an atom from one side of an equation to another side indicates oxidation. A decrease in the oxidation number of an atom indicates reduction. For example:
2AgNO3 + Cu Cu(NO3)2 + 2Ag
In this equation, on the left side of the equation the Ag has an oxidation number of silver is +1 and on the other side the Ag oxidation number is 0.
Since the silver ions decrease in oxidation number, reduction is occurring. Similarly on the left hand of the equation the Cu has an oxidation number of 0 and on the side it has an oxidation number of +2. Since the copper ions increase in oxidation number, oxidation is taking place.
Materials:
1. Reaction surface
2. Chemicals
a. Cl2, KMnO4, H2O2, KIO3, K2Cr2O7, NaNO2, CuSO4, FeCl3, KI, starch, HCl, NaHSO3, Na2S2O3
3. Pipette for stirring chemicals in reaction
4. Pencil and paper
5. Safety goggles*
Procedure:
1. Make a chart to record collected data from the experiment
2. Gather all necessary chemicals and other materials
3. Mix one drop of KI with one drop of NaOCl and observe if a reaction takes place
4. If no reaction...

Some recommendations...
With Caribbean Advanced Proficiency Examination (CAPE) and Caribbean Secondary Education Certificate (CSEC, advanced and end of secondary school examinations, with equivalence to EDEXCEL (GCE - AS A2) and GCSE, the general guidelines for writing experiments is to use present tense excluding the uses of I, He, She or etc. So one might not want to use I's, instead of saying " In the first problem, a, I wrote out the equation given in the experiment", one might want to say " In the first problem, a, the equation was written and was given in the experiment". Also when writing variables, one needs to place them under; Independent, Dependent and Constant and if that is not possible, one might want to use, Measured Variables, Constant Variables and Changed Variables. The ones included are not the only ones. For the conclusion and discussion, one needs to identify the sources of errors and these can be grouped under Random & Systematic. Also one needs to include limitations of the experiment.
The theory seems to be well researched and written accurately. Theres nothing wrong with the method, since its sort of a planing and designing experiment. But this experiment is a combination of a planning and designing and a observation experiment, as the experiment is made up with a hypothesis and that hypothesis is tested. Therefore, it is possible to use both present and past tense in the write up....
0 out of 1 people found this comment useful.