Fischer Esterification
Kyle Peterson
Chem. 243a
Matt Judd, Sec. 25
Date Performed: 10/15/03
Abstract: The objective of this experiment is to efficiently perform a fischer esterification of 1-Hexanol to form water and hexyl acetate, and to confirm the esterification with a nuclear magnetic resonance (NMR) spectroscopy. It was found that 0.3963 grams hexyl acetate was formed with a percent yield of 33.2%. The product was confirmed using NMR, IR, and boiling point confirmation.
Backround:
A Fischer Esterification is the formation of an ester and water from alcohol and an acid. More specifically it is a nucleophillic acyl substitution reaction carried out under acidic conditions. Carboxylic acids alone are not reactive enough to be attacked by neutral alcohols, but they can be made much more reactive in the presence of a strong acid, such as sulfuric acid or hydrochloric acid. The mineral acid protonates the carbonyl group oxygen atom and gives the carboxylic acid a positive charge.
Now positively charged, the carboxylic acid is much more reactive toward a nucleophillic attack by the alcohol.
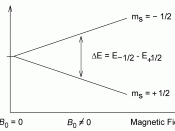
NMR (Nuclear Magnetic Resonance) spectroscopy is a technique used to determine the actual 3-D image of a molecule. This spectroscopy uses atoms that have nuclear magnetic moments such as isotope-1 Hydrogen and isotope-13 Carbon atoms. The nuclei of NMR-active atoms have one of two nuclear spin states, either (+1/2 or -1/2). These atoms when placed in a magnetic field are either spinning with the +1/2 magnetic field or against it (-1/2 spin). A slight majority of the nuclei are aligned with the magnetic field, because this spin orientation constitutes a slightly lower energy spin state. If radio frequency waves of the appropriate energy are supplied, nuclei aligned with this field can absorb this radiation and reverse their spin and now oppose the applied magnetic field. The...