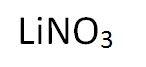Purpose:
To determine the colors of the Atomic Emission Spectra of several metallic ions.
Materials:
Safety glasses
10 test tubes
Test tube rack
Paper
Pencil
50 mL beaker
Bunsen burner
Nichrome wire
Barium Nitrate
Copper Nitrate
Strontium Nitrate
Lithium Nitrate
Potassium Nitrate
Sodium Chloride
Calcium Nitrate
Unknown solutions A,B, & C
Hydrochloric Acid
Wash bottle (with distilled water)
Procedure:
1. Label the test tubes with the names of the solutions and unknowns.
2. Place about 5 mL of each solution into each test tube, and 10 mL of Hydrochloric Acid into the 50 mL beaker.
3. Clean the Nichrome wire before each test. To do this, rinse it with distilled water from the wash bottle, dip it into the Hydrochloric Acid, and place it in the burner flame for a few moments.
4. Take note of the color of the flame when the wire is clean. This is the color you should see after each cleaning.
5. Once the wire is clean, dip it into one of the solutions, place the wire in the flame, and observe the color of the flame.
6. Clean the Nichrome wire as directed in step 3, then repeat step 5 until all of the solutions have been tested. Record your observations as you go.
7. Repeat steps 3 through 5 for each of the unknown solutions and try to determine what the solution may be based on your observations of the known substances.
Data and Observations:
Solution Color of Flame
Barium Nitrate green/yellow to orange
Copper Nitrate blue/green to orange
Lithium Nitrate purple/red to orange
Potassium Nitrate pink/purple
Sodium Chloride bright orange
Calcium Nitrate red/orange
Strontium Nitrate red to orange to yellow
Unknown A red/orange - Calcium Nitrate
Unknown B pink/purple - Potassium Nitrate
Unknown C bright orange - Sodium Chloride
The metallic ions that are present in the solutions are Barium Nitrate, Copper Nitrate, Strontium Nitrate, Lithium Nitrate, Potassium Nitrate, Sodium Nitrate, and Calcium Nitrate.
Calcium Nitrate is present in Unknown A. Potassium Nitrate is present in Unknown B. Sodium Chloride is present in Unknown C.
When the ions burn it is a chemical change.
The colors we saw and the lines of the electromagnetic spectrum are all colors of the rainbow.
Calculations:
There were no calculations made in this lab.
Conclusion:
Barium Nitrate burns green/yellow. Copper Nitrate burns blue/green. Lithium Nitrate burns purple/red. Potassium Nitrate burns pink/purple. Sodium Chloride burns bright orange. Calcium Nitrate burns red/orange. Strontium Nitrate burns red, orange, and yellow.
If other compounds containing the same metal ions were flame tested, I think that they would burn the same colors, unless mixed with others.