Chemical Basis of Life (1)
Biology is the study of living organisms. In turn, organisms are chemical machines, i.e. organisms are composed of molecules, which are collections of smaller units called atoms. Therefore, we need to consider the chemistry of life or biochemistry.
Chemical Bonding
-electrons occupy various positions (distances) from the nucleus of an atom, known as energy levels.
-as an electron moves further from the nucleus of an atom, its potential energy increases. Why? Energy is input to reduce its everyday attraction to the positively charged nucleus.
-the outermost orbital or energy levels are termed the valence orbital; the valence electrons ** the chemical behaviour of an atom is determined by the number and arrangement of the valence electrons.
The need for stability - there are a group of elements in the Periodic Table called the noble gases that are inert (non-reactive). Except for helium that has 2 valence electrons, all members of this group have 4 pairs or 8 valence electrons.
Chemical Basis of Life (2)
Therefore 8 seems to be a 'magic' number i.e. the noble gases do not attempt to gain, lose, or share electrons with other atoms. All other elements attempt to gain, lose and share electrons to achieve the ability of the noble gases. These interactions are the cause of chemical reactions that will establish the chemical bonds between atoms.
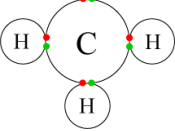
Types of Bonds
1.Ionic
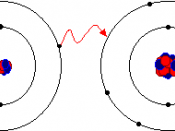
- Atoms lose electrons to become positively charged ions called cations
-atoms gain electrons to become negatively charged ions called anions.
-an ionic bond forms between metal cations and non-metal anions; it is simply electrostatic attraction involving opposite charges.
e.g. table salt - NaCl
- Sodium has 1valence electron; if it loses this, it becomes stable, resulting as the cation Na+
-Chlorine has 7 valence electrons; the addition...